Introducing NEW ConvaFoam™
Treating patients is complicated but choosing the right dressing is simple.
ConvaFoam
Wounds are a silent epidemic, with a substantial economic, clinical and social burden that is set to keep rising¹.
We have strived to create a high-performance product range that makes your dressing choice simpler.
Introducing ConvaFoam™
ConvaFoam™ is a family of advanced foam dressings designed to address the needs of you
and your patients. You can use ConvaFoam™ on a spectrum of wound types at any stage of the wound journey, making it the simple dressing choice for wound management.
Core benefits that simply stack up
Contains AQUACEL® Hydrofiber® technology
ConvaFoam™ is the only foam dressing to contain superabsorbent fibres and AQUACEL® Hydrofiber® technology
Repositionable upon application
ConvaFoam™ adhesive dressing designed to be repositionable upon application to avoid wasted dressing changes*⁵
Designed to maximise wear time
Uses silicone adhesive designed for optimal balance of adhesive strength, skin friendly adhesion and breathability*⁵
Patterned film may help aid exudate monitoring⁶
* Not applicable to ConvaFoam™ Non-adhesive dressings
AQUACEL® Hydrofiber® Technology by Convatec
* Not applicable to ConvaFoam™ Silicone Dressing
Innovation by design
Improved pore construction to enhance adhesion and absorption.
To create a dressing that offered improved clinical benefits, we enhanced the silicone in our ConvaFoamᵀᴹ Silicone and Border dressings. Our silicone adhesive has more precise and evenly distributed pores, which reduce the amount of silicone whilst improving adhesion.*5,13
*Not applicable to ConvaFoamᵀᴹ Non-adhesive dressings
ConvaFoam dressings
All ConvaFoam™ dressings can be cut⁶
All wounds are a little different, varying in shape, size, location. You need a dressing that’s versatile and can be tailored to any anatomical location.
ConvaFoam™ has been designed to offer the unique ability to cut it to the exact shape and size you need, aiding application to difficult-to-dress areas.
Conformable dressings to aid in application on body contours.6
ConvaFoamᵀᴹ adhesive dressings designed to be repositionable upon application to avoid wasted dressing changes.*5
Can be cut to aid application to difficult to dress areas.6
*Not applicable to ConvaFoamᵀᴹ Non-adhesive dressings
Improved adhesion
Improved adhesion⁵
Silicone adhesive designed to aid dressing reapplication when inspecting fragile skin and or healthy skin, when used as part of a pressure ulcer prevention protocol of care.*5,14
ConvaFoam™ adhesive dressings demonstrated to have improved adhesive strength compared to Mepilex® Border Flex, ALLEVYN Life and Biatain®.* ** 5
Silicone adhesive designed for optimal balance of adhesive strength, skin friendly adhesion and breathability to maximize wear time.*5
Silicone adhesive supports atraumatic removal.15
ConvaFoam™ Silicone protects fragile and or healthy skin from moisture, shear and friction damage when used as part of a pressure ulcer prevention protocol of care.16,17
*Not applicable to ConvaFoamᵀᴹ Non-adhesive dressings
** as demonstrated in Vitro
ConvaFoam™ as part of Wound Hygiene
Consider ConvaFoam™ as a secondary dressing when biofilm likelihood is higher
Consider ConvaFoam™ as a secondary dressing in combination with an antibiofilm primary dressing
Consider ConvaFoam™ as a primary dressing
When likelihood of biofilm is low, consider ConvaFoam™ as a primary dressing
For shallow wounds up to 0.5cm
For shallow wounds up to 0.5cm ConvaFoam™ can be used as a primary dressing
For wounds up to 2cm deep
For wounds up to 2cm deep ConvaFoam™ can be used as a secondary dressing in combination with AQUACEL® Extra™
ConvaFoam™ can be used in combination with AQUACEL® Extra™, AQUACEL® Ag, and AQUACEL® Ag Advantage™ dressings to have a synergistic effect of AQUACEL® Hydrofiber® Technology layers.
Meet the ConvaFoam™ family of dressings
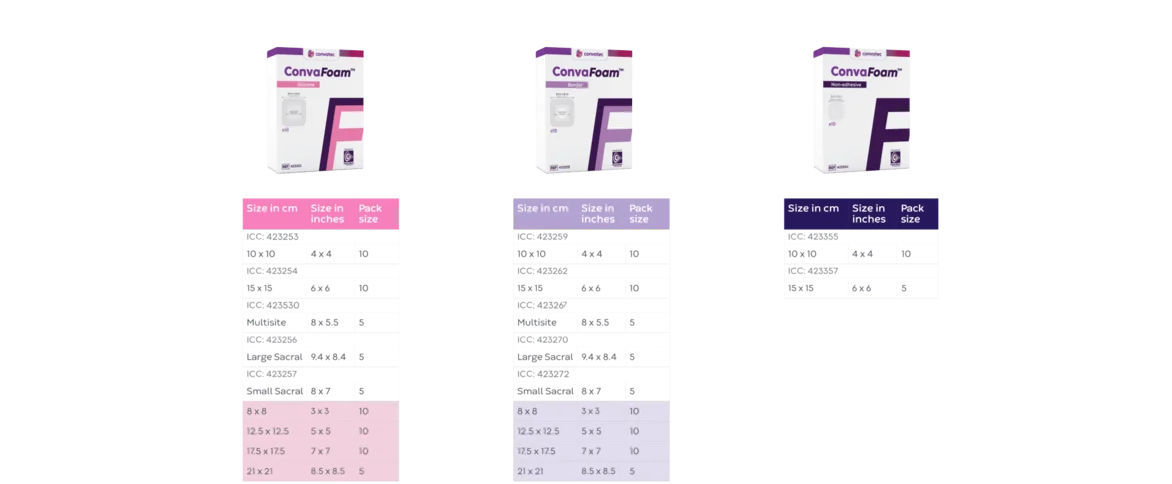 ;
;
Request a Sample
If you are a healthcare professional, please complete this brief form to request your free sample.
Have questions? Call our support team at 1-800-422-8811.
References:
Sen CK. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv Wound Care 2021 May;10(5):281-292.
2. ConvaFoam™ Silicone IFU.
3. ConvaFoam™ Border IFU.
4. ConvaFoam™ Non-adhesive IFU.
5. WHRI8050 MS172 Adhesion Characteristics of ConvaFoam™.
6. WHRI8051 MS173 In-vitro Performance Characteristics of ConvaFoam™.
7. Jones S, Bowler PG, Walker M. Antimicrobial activity of silver‐containing dressings is influenced by dressing conformability with a wound surface. WOUNDS. 2005;17(9):263‐270.
8. Hoekstra MJ, Hermans MH, Richters CD, Dutrieux RP. A histological comparison of acute inflammatory responses with a hydrofibre or tulle gauze dressing. J Wound Care. 2002;11(3):113‐117.
9. Bowler PG, Jones SA, Davies BJ, Coyle E. Infection control properties of some wound dressings. J Wound Care. 1999;8(10):499‐502.
10. Walker M, Hobot JA, Newman GR, Bowler PG. Scanning electron microscopic examination of bacterial immobilisation in a carboxymethyl cellulose (AQUACEL®) and alginate dressings. Biomaterials. 2003;24(5):883‐890.
11. Waring MJ, Parsons D. Physico‐chemical characterisation of carboxymethylated spun cellulose fibres. Biomaterials. 2001;22(9):903‐912.
12. AQUACEL® Foam & AQUACEL® Ag Foam Product Monograph.
13. ConvaFoam™ Pore Size WHRI8196 MS176.
14. Healthy Volunteer Study, CNVADH2F, 04 August 2022.
15. Soft Silicone Dressings Made Easy Meuleneire F, Rücknagel H Wounds International May 2013.
16. WHRI8052 MS174 ConvaFoam™ Dressing Characteristics for the use in Skin Protection.
17. European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. The International Guideline. Emily Haesler (Ed.). EPUAP/NPIAP/PPPIA. 2019. [p22].
18. Murphy C, Atkin L, Vega de Ceniga M, Weir D, Swanson T. International consensus document. Embedding Wound Hygiene into a proactive wound healing strategy. J Wound Care 2022;31:S1–S24.